Potassium Permanganate - Introduction
Potassium Permanganate is an inorganic compound, it is also known as Condy's Crystals, Potassium Manganate (VII) or Permanganate of Potash.
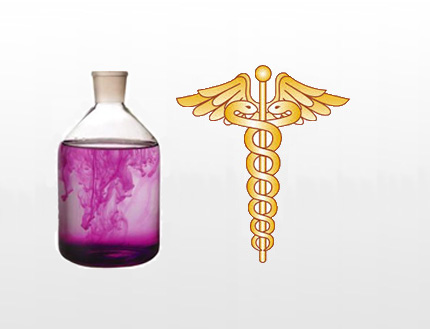
Potassium Permanganate is a dark purple crystalline inorganic compound, it is also been known as in the past as Condy's Crystals, Potassium Manganate (VII) or Permanganate of Potash. It has the appearance of dark purple crystals. It was first prepared in the 17th Century by a German-Dutch Chemist Johann Rudolf Glauber by fusing a mixture of Potassium Carbonate and Manganese Dioxide, forming Potassium Manganate, which when in solution turned from green to purple, giving aqueous Potassium Permanganate. Most of Potassium Permanganates applications relies on its oxidising properties - Uses and Applications are covered in the next slider bar
On an industrial scale Potassium Permanganate is produced by fusing Pyrolusite (a naturally occurring mineral containing Manganese Dioxide MnO2) with Potassium Hydroxide (Caustic Potash), heated with air, or introducing oxygen from oxidisers such as Potassium Chlorate or Potassium Nitrate. This produces Potassium Manganate, which with electrolytic oxidation within an alkaline media or by heating the Potassium Manganate solution to boiling in the presence of Carbon Dioxide (CO2) until the green solution changes to purple giving Potassium Permangante. Any excess insoluble Manganese oxide is filtered off and the Potassium Permanganate is crystallised out by evaporation.
Potassium Permanganate - Images
Crystalline (Needle Crystals) Crystalline (Fine Crystals) Solution (Different Stages of Diffusion)